An important note relating to this data – the assays used across the trials were not standardized. This means we can only compare the data across the dosing in each trial, and not the data from one trial to another. To put this another way, a GMT of 1064 in the Geneva trial doesn’t necessarily suggest an improved response over the GMT of 1300 in the Washington trial.
Bottom line here is that this one looks good from an efficacy perspective, but only insofar as it was trying to prove that an immune response could be generated, not necessarily that that immune response would be effective against live Ebola virus.
Aside from Phase 1 trials, there are two primary ongoing phase 3 studies sponsored by Merck & Co., Inc. (NYSE:MRK) in West Africa. Interim data published last year suggested a 100% efficacy rate – i.e. all individuals that received the vaccine achieved immunity to the “fake Ebola” within 6-10 days. The p value on the data was 0.0036, which is great from a statistical significance perspective. The FDA generally requires a p value of .05 or less. In other words, it seems this vaccine works well, at least for what it was trying to prove.
ChAd3-EBO-Z
ChAd3 vaccines are currently under investigation across a range of glycoprotein expressions, with the two primary being the Zaire glycoprotein and the Sudan glycoprotein. The one we are interested in here is the Zaire GP, as this is the most sought after approach. The vaccine was developed by the Vaccine Research Center of the National Institute of Allergy and Infectious Diseases, and picked up by GlaxoSmithKline plc (ADR)(NYSE:GSK) back in May, 2013. The data from a completed Phase I trial is illustrated in the table below. The table also includes data from a completed Phase I in a vaccine that is bivalent – that is, it contains both the Zaire and the Sudan GPs. The mono vaccine, the one listed in the table as EBO-Z also taken from the FDA briefing document, is the vaccine that GSK is developing.
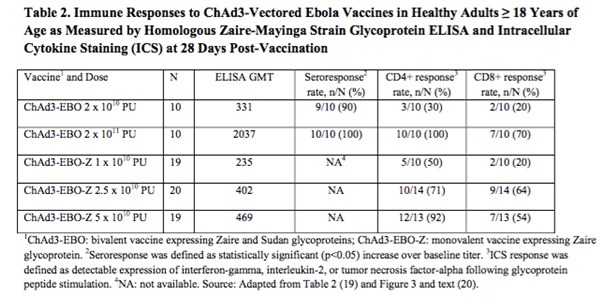
As the data in the lower three rows in the table shows, the response to this vaccine was similar to that of the previously discussed rVSV-ZEBOV, in that the higher the dose concentration, the higher the response as measured by ELISA and recorded as GMT. Seroresponse data from this trial is not available, which weakens the dataset a little bit, but this should be resolved when we get later stage data.
Note that the immunogenicity is not as robust at the highest mono dose (GMT = 469) as it is in the highest bivalent dose (GMT = 2037). Unlike the previously discussed trial, these are comparable, and this indicates that the bivalent is probably more effective than the mono. This, again, weakens the GlaxoSmithKline plc (ADR)(NYSE:GSK) candidate a little. In the interest of balance, however, these results were taken at the 28-day post administration point. If GSK can demonstrate that the GMT increases after this point, or extends beyond the bivalent vaccine in terms of how long the immune response maintains, it would strengthen the bid for commercialization.
A GSK study is currently ongoing for this vaccine, and if it completes successfully the vaccine will be fast-tracked for approval and shipped to West Africa. The company has reportedly already prepared and is storing ten thousand doses in anticipation of an approval.





