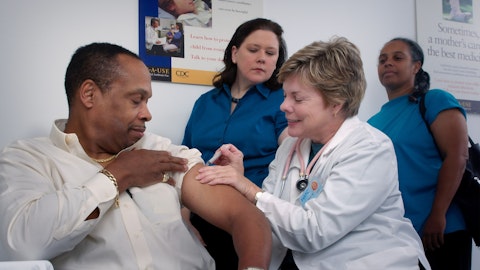
Over the coming months, Mene and Sharon will complete an extensive handover, supporting a smooth transition of responsibility and ensuring there are no delays to the advancements of projects, which brings me to Mene, someone who’s always quick to praise others, but must not take credit himself for all it has done to transform how we approach R&D, delivering a greater than five-fold improvement in productivity, driving deeper collaborations with academic, biotech and key organizations, pioneering programs to promote open innovation and championing the use of new technologies and mortality. And last but not least, leading our contribution to the U.K. life sciences sector and in particular, building a very important presence in Cambridge in the U.K. Mene, thank you for your contribution to our company, and I wish you, only the best in your retirement.
Personally, I will miss your wonderful sense of humor and your intellectual with. But the quality of the medicines you brought to patients and the pipeline and capabilities you’ve built will be your legacy for many years to come. With that, I will hand the call back to Andy, for our Q&A session.
A – Andy Barnett: We will now go to the Q&A with all of our executive members participating shown here. As a reminder, you can raise your hand on Zoom or type your questions in via the Q&A button. We will try and answer as many questions as we can during the call, but please do limit the number of questions you asked to allow others a fair chance to participate in the Q&A. And with that, we move to the first question.
Pascal Soriot: Sachin Jain, Bank of America. Over to you Sachin.
Sachin Jain: Thanks for taking my questions. It’s Sachin Jain from Bank of America. Two topics, firstly, Dato in first-line lung, if I may so part of the reaction to TROPION-Lung01 was investor fading confidence in first line on both efficacy and safety, so I wonder if you could touch on that. And could you remind us how you interpret the eight months of PFS we saw from TLA2 in ASCO just the competitor, [Indiscernible] nine months. And if Susan, you could frame expectations of what our focus should be into TL04 that you flagged at word Lung? And then the second question is on breast cancer, a lot of news flow in breast cancer in the next 12 months. So we’ve been particularly focus, so I wonder if you could just touch on two reads.
Firstly, TB01 which you talked about but the potential for that to be better than TROPICS-02, you noted the eight-month PFS, are you confident that can repeat and better the trade by 5.5 months? And then secondly, you didn’t mention DB09, but you pulled that forward into 2024. So again, just talk to the confidence you’ve got there, prior data suggest potential for double the PFS comparator receptive data? Thank you.
Pascal Soriot: Thanks, Sachin. So easy question for you, Susan?
Susan Galbraith: Thanks for the question, Sachin. So let’s start with data in first-line lung. First of all, I would say that given the TLO1 is a positive study, there were things that we can learn from that. The first-line lung studies are ongoing. We’ve, obviously, got TROPION-Lung07, 08 and AVANZAR, which are complementary trials in first line, predominantly in patients without genomic alterations versus the respective standard of care. AVANZAR is investigating patients regardless of PD-L1 status of gene histology. So they’re complementary segments of first-line lung. What I would say is that we’ve now treated across all of these studies, together with TLO2 and TLO4 over 200 patients on the Dato-DXd plus immune checkpoint inhibitor combination.